38 open-label study disadvantages
Effects of open-label placebos in clinical trials - Nature by M von Wernsdorff · 2021 · Cited by 35 — Our study has some limitations. We did not explicitly search for grey literature, like unpublished but completed studies, dissertations, and ... Open-label extension studies: do they provide meaningful ... by RO Day · 2007 · Cited by 39 — Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for ...
What is an Open-Label Clinical Trial? - News Medical 31 Mar 2022 — Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled, this data is not of ...

Open-label study disadvantages
Official journal of the American College of Gastroenterology ... The American Journal of Gastroenterology is pleased to offer two hours of free CME credit with each issue of the Journal.This activity is the result of our reader survey that expressed great interest in journal CME. Reducing bias in open-label trials where blinded outcome ... by BC Kahan · 2014 · Cited by 75 — For objective outcomes, such as all-cause mortality, unblinded assessment is unlikely to bias the trial results [5]. However, evidence suggests ... limitations of open-label studies assessing antiangiogenic ... by JC Trone · 2018 · Cited by 8 — Open-label trial design may lead to an overestimation of an exiting risk of adverse drug effects, or even detect which do not exist. For more accurate estimates ...
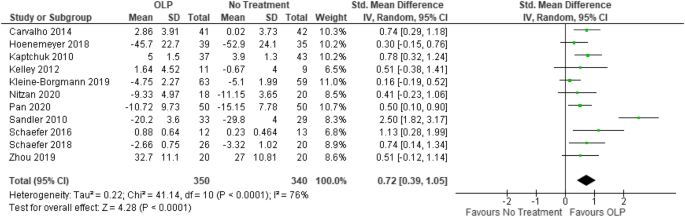
Open-label study disadvantages. Analysis of clinical trials - Wikipedia The assumption is that the patients improve gradually from the start of the study until the end, so that carrying forward an intermediate value is a conservative estimate of how well the person would have done had he or she remained in the study. The advantages to the LOCF approach are that: It minimises the number of the subjects who are eliminated from the analysis, and; It … (PDF) What is an open label trial? - ResearchGate 5 Jul 2022 — Another limitation is linked to the open trial design, where participants are aware of their group allocation, thereby the risk of potential ... External and internal validity of open label or double‚•'blind ... by J BEYER‐WESTENDORF · 2011 · Cited by 61 — disadvantages of open-label or double-blind trials is currently under way and interpretation of trial results is often focused on this matter. Guidance on use of Imvamune in monkeypox outbreaks in Canada ... Aug 03, 2022 · In a Phase 3 Footnote 11, randomized, open-label active-controlled non-inferiority trial, 440 smallpox vaccine naïve adults were randomly assigned to receive 2 doses of Imvamune ® 4 weeks apart followed by one dose of a second generation replicating smallpox vaccine (to observe for effect of Imvamune ® on vaccinia vaccine cutaneous reaction ...
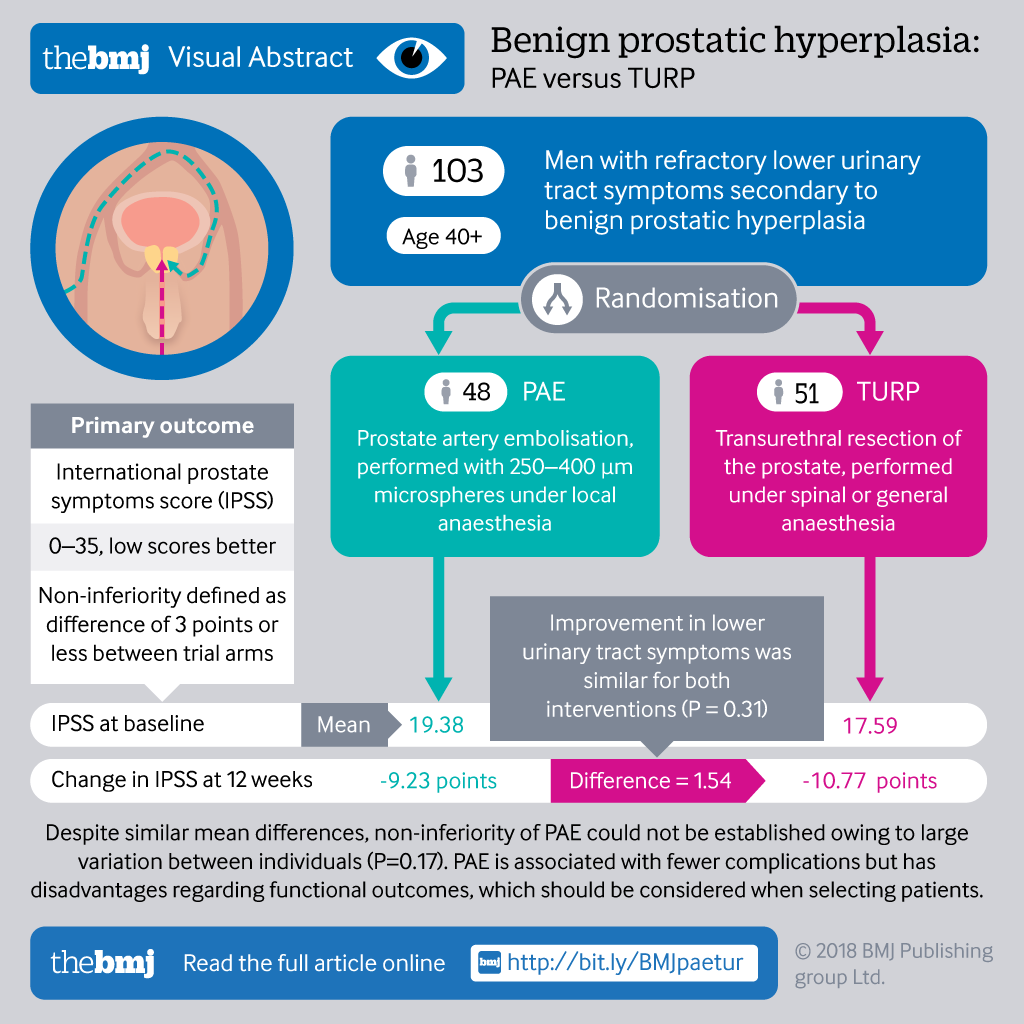
Randomized controlled trial - Wikipedia A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments. Phage therapy - Wikipedia Phage therapy, viral phage therapy, or phagotherapy is the therapeutic use of bacteriophages for the treatment of pathogenic bacterial infections. This therapeutic approach emerged at the beginning of the 20th century but was progressively replaced by the use of antibiotics in most parts of the world after the second world war. Open-label trial - Wikipedia An open-label trial may still be randomized. Open-label trials may also be uncontrolled (without a placebo group), with all participants receiving the same ... UpToDate Sep 08, 2022 · Reznik Y, Cohen O, Aronson R, et al. Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet 2014; 384:1265. Meade LT, Battise D. Evaluation of Clinical Outcomes With the V-Go Wearable Insulin Delivery Device in Patients With Type 2 Diabetes.
Amphotericin B: Package Insert / Prescribing Information ... Feb 01, 2022 · Amphotericin B Description. Amphotericin B liposome for injection is a sterile, non-pyrogenic lyophilized product for intravenous infusion. Each vial contains 50 mg of Amphotericin B, USP, intercalated into a liposomal membrane consisting of approximately 0.64 mg alpha tocopherol; 52 mg cholesterol; 84 mg distearoylphosphatidylglycerol, sodium salt; 213 mg hydrogenated soy phosphatidylcholine. limitations of open-label studies assessing antiangiogenic ... by JC Trone · 2018 · Cited by 8 — Open-label trial design may lead to an overestimation of an exiting risk of adverse drug effects, or even detect which do not exist. For more accurate estimates ... Retinoids in the treatment of skin aging: an overview of ... Jan 04, 2006 · Considering the RAR binding activity of alitretinoin, Baumann and colleagues (2005) recently conducted an open-label pilot study in 20 patients with photodamaged skin to evaluate the efficacy of 0.1% topical alitretinoin. The topical alitretinoin treatment showed improvement in seborrheic keratoses, actinic keratoses, and other symptoms of ... limitations of open-label studies assessing antiangiogenic ... by JC Trone · 2018 · Cited by 8 — Open-label trial design may lead to an overestimation of an exiting risk of adverse drug effects, or even detect which do not exist. For more accurate estimates ...
Reducing bias in open-label trials where blinded outcome ... by BC Kahan · 2014 · Cited by 75 — For objective outcomes, such as all-cause mortality, unblinded assessment is unlikely to bias the trial results [5]. However, evidence suggests ...
Official journal of the American College of Gastroenterology ... The American Journal of Gastroenterology is pleased to offer two hours of free CME credit with each issue of the Journal.This activity is the result of our reader survey that expressed great interest in journal CME.













![PDF] Clinical trial methodology to assess the efficacy ...](https://d3i71xaburhd42.cloudfront.net/a00f5af9933a8f9c61da678199905782adf4210d/8-Table4-1.png)
![PDF] The Clinical Viewpoint: Definitions, Limitations of ...](https://d3i71xaburhd42.cloudfront.net/0d341492d445073e64d1ccb6985e4ad763a3d1f7/2-Table1-1.png)









Post a Comment for "38 open-label study disadvantages"